We’ve been discussing the importance of an emerging biopharma company’s supply chain team in our recent blog series, “Heroes in the Commercialization Journey”. We introduced 3 common problems we see arise in areas of a new product’s supply chain. The first topic centered on common problems in the upstream supply group. This second topic involves the downstream product supply group and how timing of hiring Trade and Distribution leadership influences commercial success.
Hiring Trade and Distribution Too Late to Plan and Execute the Downstream Supply Chain
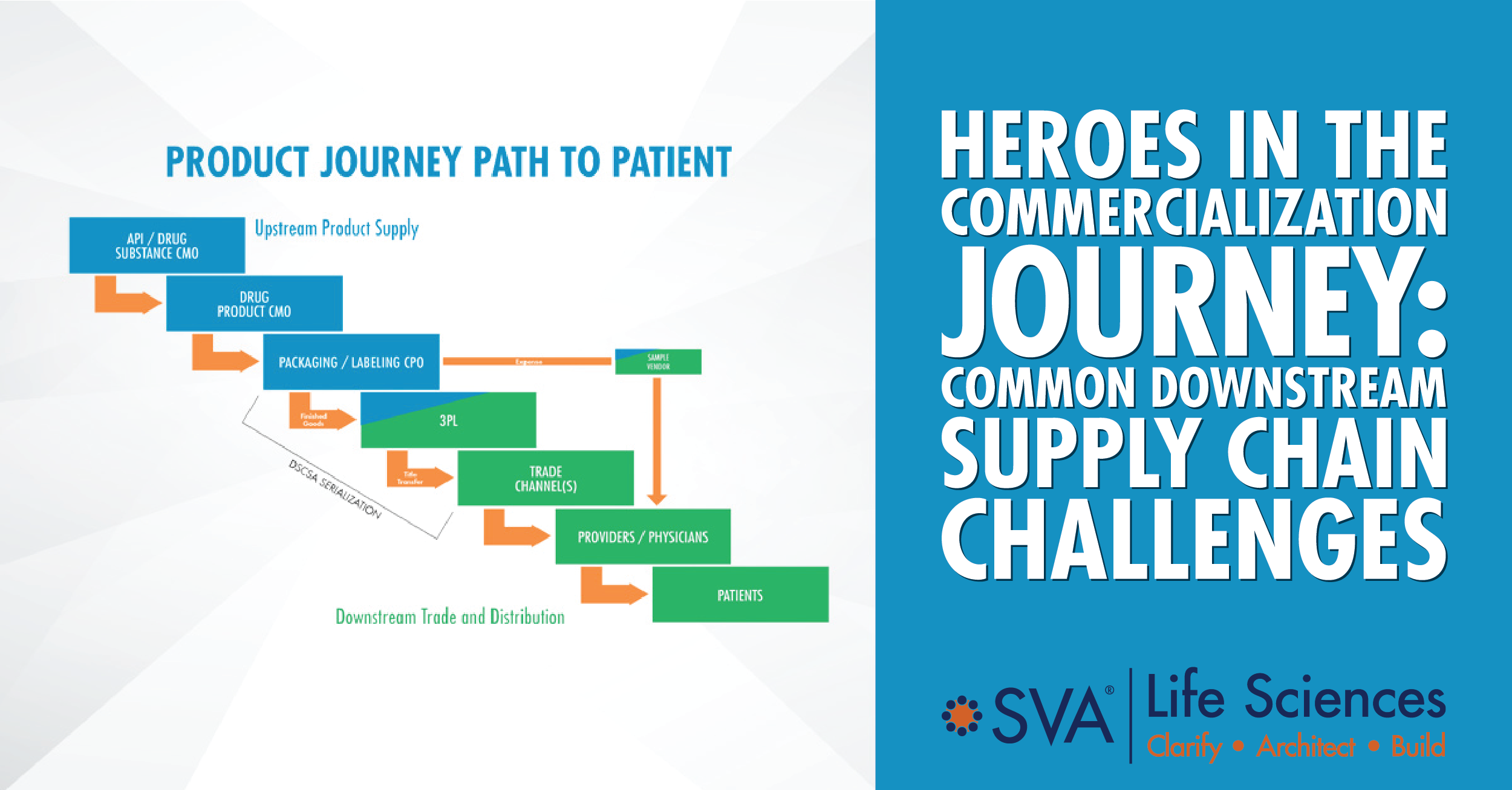
The downstream supply chain requires an efficient distribution network, and the optimal window for hiring critical personnel is short. This area of the supply chain is led by Commercial’s Trade and Distribution (T&D) function, and it begins with a third-party logistics (3PL) partner that executes the order-to-cash process. From the 3PL, product enters a myriad of distribution options that have to be configured accurately and efficiently to address the very unique combination of product, prescriber, payer, pharmacy, provider, and patient characteristics to meet unmet needs.
A head of Market Access should be hired at least 24-30 months from PDUFA. Mastery of essential distribution functions is the secret ingredient to ensuring patient and commercial success, but that requires leadership in crucial planning and long lead-time activities. The most effective and efficient distribution network implementations can answer questions early on about where patients will receive the drug (hospitals, clinics, physician’s offices, etc.), what defines the current market dynamics and what the plan is for tracking and analyzing them as they evolve (pricing, formulary positioning, rebating, value-based contracting, patient services), and whether product technical parameters (temperature controls, expiration dating, half-life) and/or target patient populations (micro/orphan) require special design features.
The head of Market Access should be experienced in the therapeutic area and able to lead efforts to develop a compelling product value proposition supported by robust clinical and economic evidence with geographic responsibility. This role is responsible for crafting the access and distribution strategy and recruiting a talented team to deliver on that strategy and should be familiar with the fundamentals of establishing supply chain functions from the ground up. When the optimal hiring window for downstream supply chain leadership roles shortens, options to mitigate are compressed and things can get expensive. In addition, without timely leadership, you risk missing the ultimate goal: the product getting to patients.
Do these challenges sound familiar to you? We want to hear your feedback, and we welcome any questions you may have on this topic.

© 2021 SVA Life Sciences