A repeatable and sustainable approach to getting commercial product to patients.
A biopharma company’s supply chain team are often the unsung heroes of commercialization – when everything goes as planned. Fusing all the moving parts, from confirming the quality and quantity of the product to ensuring its channel distribution is on time, is a herculean undertaking.
The three most common questions asked are:
“How do we ensure that quality product will be in channel on time?”
“How do we meet demand and expiration requirements?”
“What are our weak links and knowledge gaps?”
We have experience establishing commercial supply chains for over 50 products providing a robust, economically feasible, and predictable assessment and planning methodology that not only serves the organization now, but intentionally considers the future.
Commercialization Journey
At this stage of the commercialization journey you’re about a year away from FDA approval. You’ve gone through the commercialization and organizational readiness phases and are now ready to build out the product journey path to patient by mapping out all facets of your supply chain.
What you should be focused on at this stage of the commercialization journey:
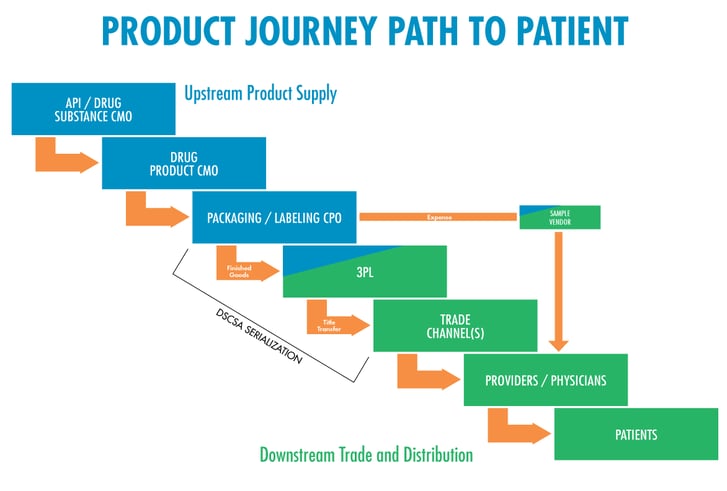
A comprehensive supply chain strategy considers both the upstream product supply and the downstream trade and distribution (see above chart) and is comprised of emerging best practices, identifying gaps, and creating plans to get ahead of potential challenges.
Our empirical knowledge gained from working with diverse companies and challenges enables us to develop a roadmap that optimizes your operations while ensuring your supply chain management is seamless.
Taking the time to design the ‘path to patient’ before implementation de-risks the inherent obstacles to success. Despite a team’s clinical supply experience, problems can surface in early stages out of a lack of familiarity with the nuances and complexity of a commercial business.
Our solutions offer a practical approach to selecting fit-for-purpose partners and systems.
Focusing on the design process before implementation ensures all the individual components work in concert with each other and there are no outliers to success. This results in the timely delivery of product in channel, cross-functional processes, third-party vendor support, and the management and buildout of the Supply Chain function.

contact@svalifesciences.com

1221 John Q Hammons Dr., Suite 201, Madison, WI 53717

1600 Utica Ave S, 9th Floor,
St. Louis Park, MN 55416

109 West Commercial Street, Suite 107, Sanford, FL 32771

(800) 366-9091

18650 W. Corporate Dr., Suite 200, Brookfield, WI 53045

7135 E. Camelback Road, 230, Scottsdale, AZ 85251
©2026 SVA Consulting, LLC. All Rights Reserved. | Privacy Policy | Cookie Policy | CCPA