Getting a first drug product approved is an exciting, promising milestone in an emerging biopharma company launching its first product, but you need to successfully get the therapy to patients for real success.
A biopharma company’s supply chain team is often the unsung heroes of commercialization – when everything goes as planned, that is. This means making sure the right quantities are safely and reliably in channel after approval, so the company can operate as a viable commercial entity.
Simple, right? Not at all. The supply chain involves multiple functions that likely have not had to collaborate before on a topic as complex and nuanced as a biopharmaceutical supply chain.
The Product Journey
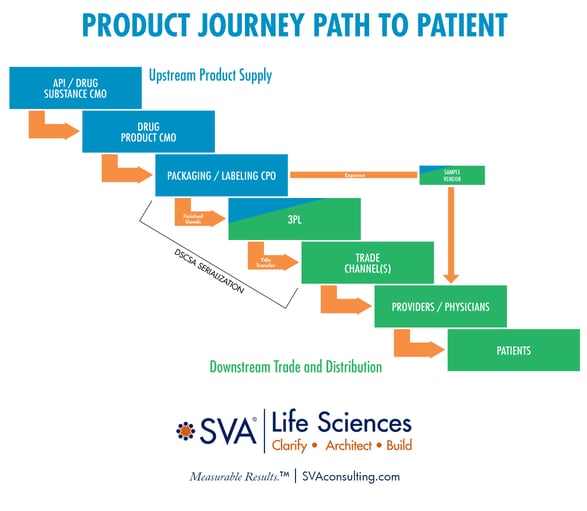
The Product Journey, or path to patient, originates with the raw materials and drug substance that is converted into the drug product and then packaged and labeled as a finished good. We refer to this as the upstream supply chain (see diagram below) which is led by Technical Operations. The manufacturing process is as complex as today’s innovative therapies demand but amplified by extensive reliance on many third parties to manufacture, test, and package the finished goods. The process integration, coordination, and intense amount of problem-solving required can be daunting.
Next, the product enters the downstream supply chain which is led by Commercial’s Trade and Distribution function. It begins with a third-party logistics (3PL) partner who executes the order-to-cash process. From the 3PL, the product enters a myriad of distribution options that need to be configured to address the unique combination of product, prescriber, payer, pharmacy, provider, and patient characteristics that must be resolved to address unmet needs and achieve commercial success.
We’ve found that clients who are contemplating commercialization of their first product want to know emerging best practices, what they may be missing, and how to get in front of challenges. We work with a range of stakeholders. Most often, Chief Commercial Officers who are accountable for commercial performance that depends on product in channel, and Supply Chain leaders who are accountable for successful planning and execution of the processes that deliver product in channel.
Problems can arise in the upstream and downstream supply chain as well as when stakeholders from functional groups need to collaborate. We recognize 3 common themes that are the root cause of supply chain challenges – these are preventable issues that can mean a major interruption in a newly approved product’s path-to-patient.
3 Common Themes at the Root of Supply Chain Challenges:
1. The Upstream Product Supply Group Lacks Commercial Experience
The clinical supply or CMC team has been working to provide product for clinical trials. However, they often lack experience and understanding of nuances and complexity of the commercial supply chain such as chain of custody, import/export, quality management systems, demand and supply planning, product serialization, pre-approval inspection requirements, and more.
2. Hiring Trade and Distribution Too Late to Plan and Execute the Downstream Supply Chain
Mastering trade, distribution, access, and reimbursement are critical ingredients for ensuring patient and commercial success. There are crucial planning and long lead-time activities that need to be led by a downstream supply chain leader who is experienced both in the therapeutic area and with establishing downstream supply chain capabilities from scratch.
3. Downstream and Upstream Disconnect
A disconnect occurs when processes and workstreams haven’t been properly mapped out to provide a holistic picture with clear roles and responsibilities between functions. Without a holistic view, you lack a cohesive way to bring stakeholders together, especially when the pressure increases closer to approval.
Each of these themes presents challenges and risks to a successful (and what should be exciting!) commercialization journey.
This is the first of a series of posts on this topic that will explore each of these three problems, and the ways we avoid the risks they present using effective solutions. Subscribe on the right-hand side of this page to be notified when we post. We’re happy to share what we know and have learned – contact us if you have a specific question.

© 2021 SVA Consulting

