We recently discussed the importance of an emerging biopharma company’s supply chain team, and the reasons we consider them the unsung heroes of commercialization. A company may have an incredible product, but it won’t change lives unless it gets into the hands of patients. In our previous post, we introduced 3 common problems we see arise in areas of a new product’s supply chain functions. Let’s spend some time unpacking the first challenge involving the upstream product supply group.
The Upstream Product Supply Group Often Lacks Commercial Experience
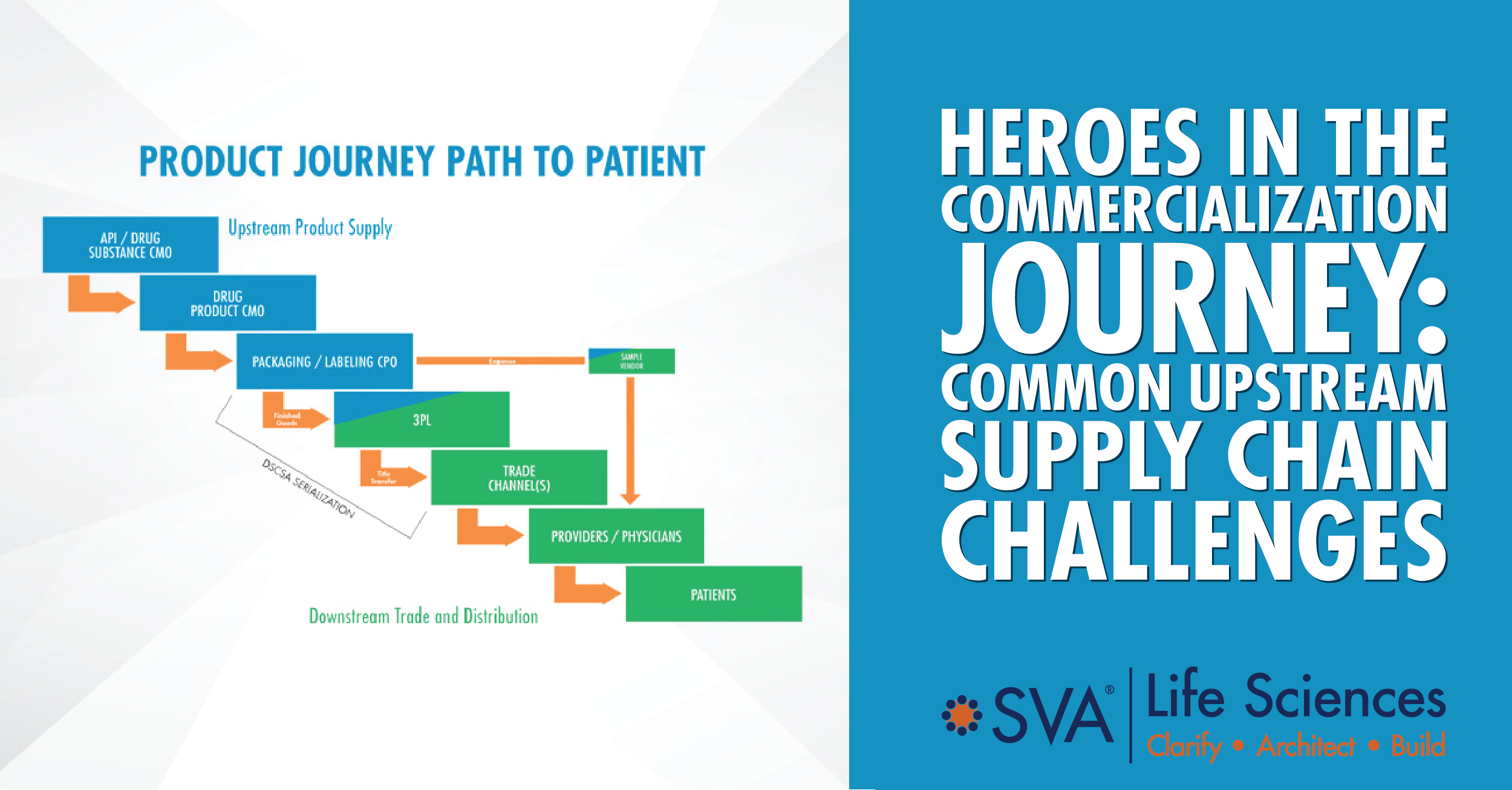
The upstream supply chain, led by Technical Operations, handles the origination of the product in its journey to the patient. Raw materials and the drug substance are converted into product, then packaged and labeled as a finished good. This manufacturing process is highly complex, as today’s innovative therapies are, but even more so because of reliance on third parties to manufacture, test, and package the finished goods. This requires precise planning, process integration, and an intense amount of problem mitigation.
Despite the CMC team’s clinical supply experience, problems can surface out of a lack of familiarity with the nuances and complexity of the commercial aspects of supply chain. Scheduling, logistics, timing issues, and trade-offs need to be considered when designing upstream operations, most of which involve entirely new responsibilities for a CMC team. Early on, this team is expected to answer some of the most critical questions in supply chain development; questions around 5-10 year product unit forecasts for all anticipated markets and partners, physician sampling, capacity of virtual manufacturers to supply new product forecast demands, conversion of clinical trial patients to use of the product, and target product profile specification sufficiency... and that’s the short list.
Leadership must be proactive in readying and supporting these teams. The upstream supply chain needs to develop necessary processes, plans and capabilities earlier than other functions, which means they may need extra support by leveraging internal or even external expertise.
The solution to these challenges is found in an organization’s ability to get ahead by mapping out their processes, identifying where their team needs help, and dodging probable obstacles. That said, appearances of overconfidence don’t ensure harmony and coordination. Watch out for warning signs of this, like language that indicates there is “no need to worry” or “we have it under control”. Leaders should create an environment that encourages open discussion, debate and bringing issues to light rather than stifling them. We live by the maxim, “keep it to yourself it’s your problem - share it with the group it’s our problem.”
Do these challenges sound familiar to you? We want to hear your feedback and we welcome any questions you may have on this topic.

© 2021 SVA Life Sciences